
pH is only one of several factors that determine soil and plant health, but left unidentified, it could be seriously detrimental to your revegetation efforts.
Studies show that soil pH varies widely by location across Australia, affected by factors such as leaching and the presence of vegetation and basic rocks, all of which can have a significant impact on soil quality. While addressing pH is critical, many worksites find that their workflow does not allow sufficient time for a soil conditioning program, leading to revegetation failures.
Are you concerned about the impact of pH levels on your site soils? If so, read on to find out more about understanding, testing and correcting soil pH levels.
Defining soil pH
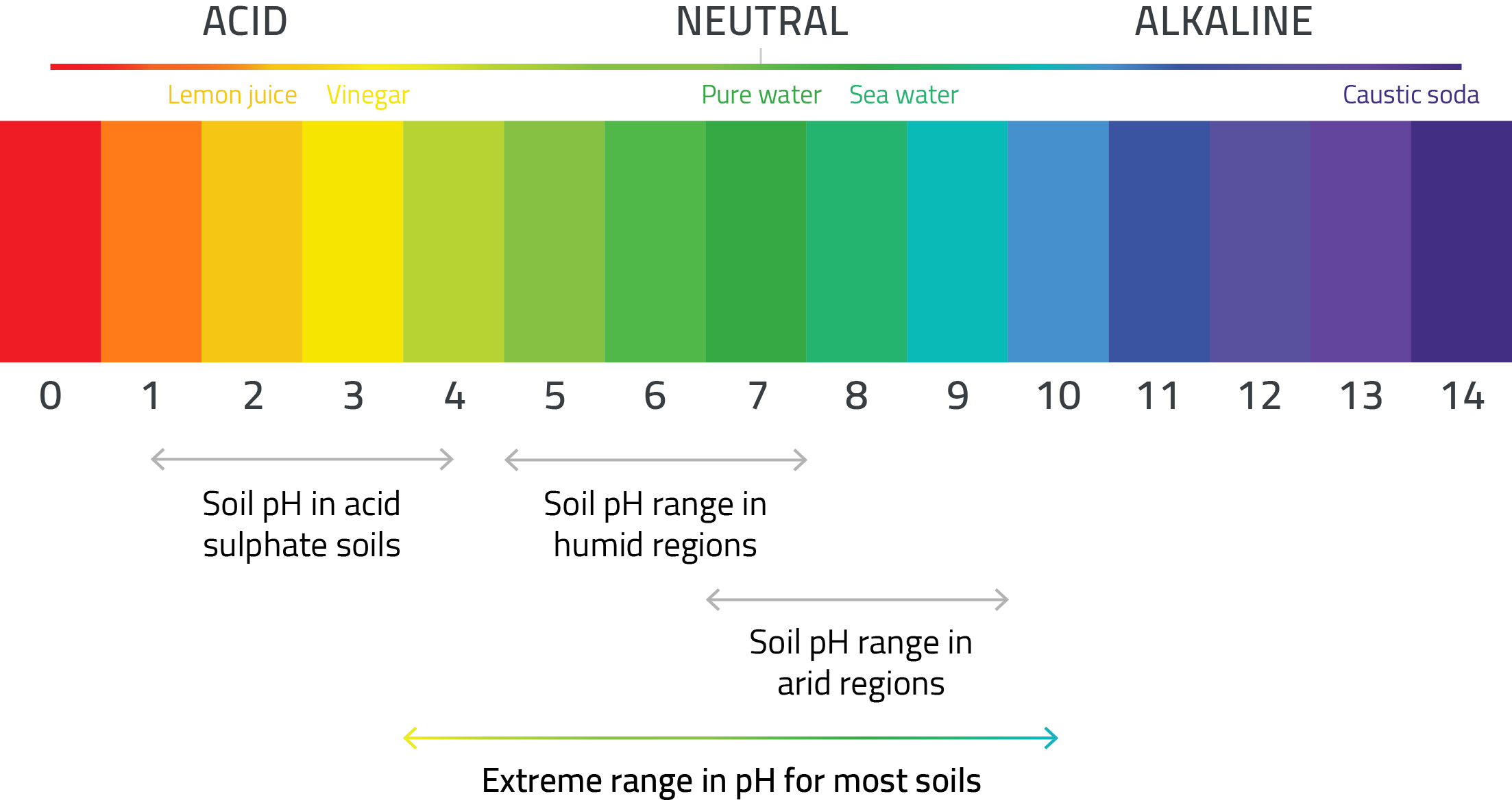
Soil pH is a measurement of the acidity or alkalinity in a soil. pH is defined as the negative logarithm of the activity of hydronium ions in a solution. In water, it normally ranges from -1 to 14, with 7 being neutral.
There are two common testing methods for soil pH:
- Using a pH testing kit.
These can be purchased from local hardware supply stores. - Sending soil samples to a laboratory.
For optimum data scope and quality, it’s best to send soil samples to a NATA-approved lab. This option also allows for a wide range of soil characteristics and mineral concentrations to be tested.
A soil pH between 6.4 and 7.4 indicates optimum availability of water-soluble nutrient minerals for plant root uptake. However, this also indicates that these minerals are most susceptible to volatilisation and most likely to react with each other and/or leach out of the soil profile. The more the soil pH deviates from this optimum range, the more these reactions take place and the less available they are for plant root uptake. This phenomenon explains why it’s so important to balance soil pH with soil conditioners in conventional farming systems.
Understanding soil conditioners
Soil conditioners are important components of maintenance and fertilisation programs – and the most appropriate soil conditioner depends on the soil analysis. Low soil pH levels can be conditioned with lime and dolomite, while high levels are typically conditioned with Gypsum, iron sulphate and other sulphate-based fertilisers.
The BioGrowth™ system applies these same minerals in non-water soluble form, meaning soil pH and associated chemical reactions, volatilization and leaching factors are not at play. Therefore, soil pH is essentially irrelevant. The soil biology species mine for these nutrient minerals as a source of energy and simultaneously exchange them with plants for root exudates if and when required.
The use of soil conditioners like lime dolomite and Gypsum in water-soluble fertiliser systems is critical, and even more so during periods of intense rainfall due to the excess leaching of nutrients. This process can cause plant nutrient deficiencies and induce conditions that lower soil pH.
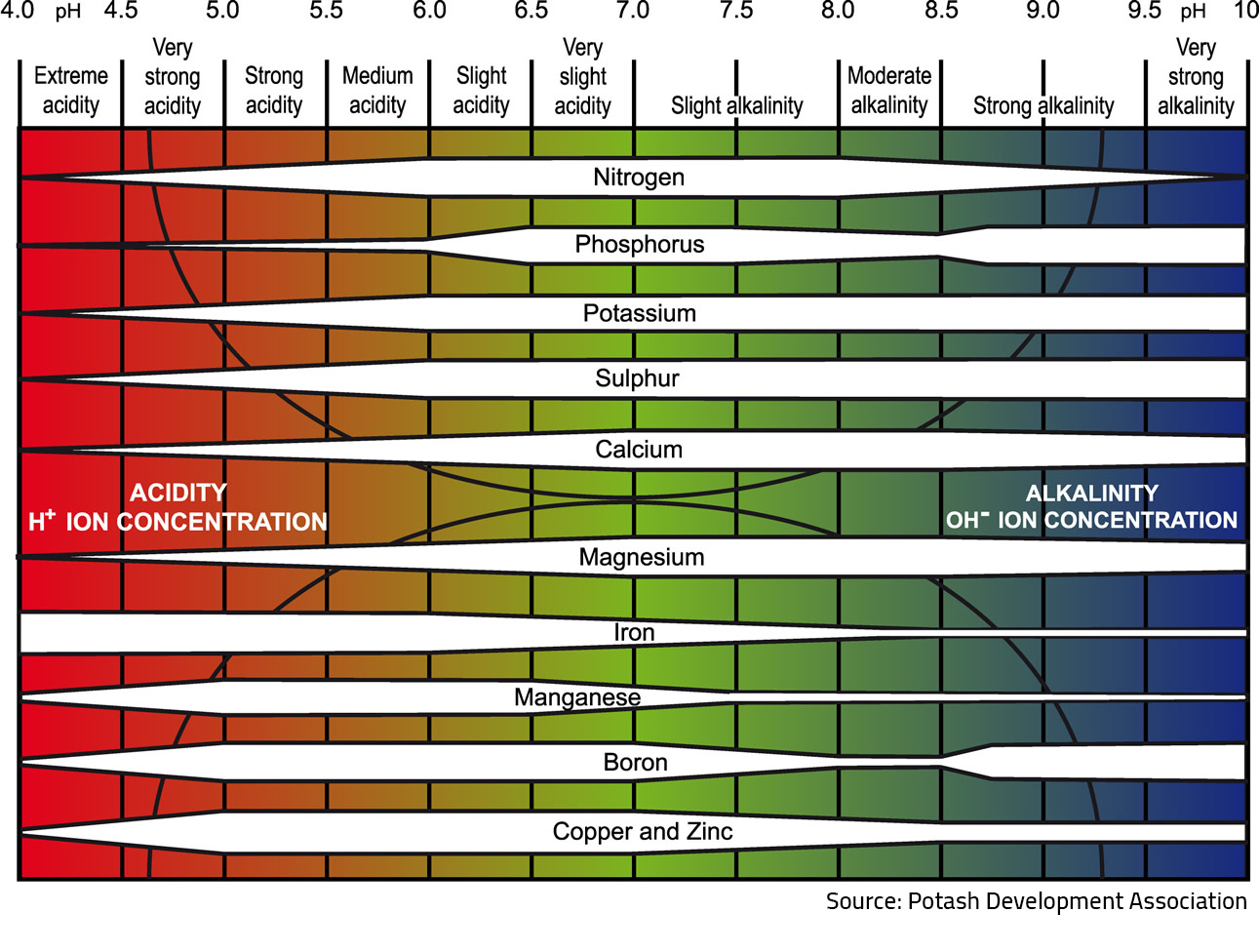
Measuring total mineral levels is important to ensure that suitable levels of each mineral are available once the soil biology system reaches peak performance. The following soil pH chart shows the plant-available nutrient (water-soluble) levels at various soil pH levels.
Correcting soil pH
While the principles and practices of the BioGrowth™ system don’t recommend specifically correcting soil pH, they do recommend soil conditioners that address mineral deficiencies developed under specific climatic conditions.
The correct process for adjusting soil pH levels depends on two factors:
- Test results
In conventional farming systems, lime and dolomite are commonly used to correct low soil pH levels. Common treatments for high soil pH include Gypsum and iron sulphate and/or acidifying water soluble fertilisers. - Soil texture class
Soil texture can alter the effect of applied soil conditioners significantly. pH will increase more in sandy soils with low buffer capacity than in clay soils. For example, applying one ton of high-quality lime may increase a sandy soil pH by 0.3 and a clay soil by only 0.15.
In the roading, construction and mining industries, these same soil conditioners are also used to enhance physical characteristics of soil like stability, resulting in a more dense, compacted soil post-earthworks.
Like any other soil conditioner, Calcium Plus (and Carbon Plus, to a lesser extent) may impact soil pH if applied at well above recommended rates. However, these products are primarily designed to supply missing minerals rather than to alter soil pH.
Common pH misunderstanding
Excessive use of soil conditioners is one of the most common challenges when it comes to correcting soil pH – particularly in the revegetation industry. Applying large amounts of soil conditioners is not conducive to timely, long-term stabilisation of reclaimed land after major earthworks. While this may enhance some soil characteristics, such as stability and structure, it may also induce mineral imbalances and nutrient lock up constraints that affect vegetation establishment post-conditioning.
The BioGrowth™ program approach is different in that it is designed to work with nature in a more holistic and natural way. This approach targets soil microbiology development based on an assessment of the soil and climatic conditions. Most importantly, the recovery and stabilisation process is driven not by soil conditions but by the symbiosis between soil biology species and selected plant species.

When did you last have your onsite pH levels tested? If your site soils are due for a checkup or a mineral adjustment, reach out to our soil scientists and find out how BioGrowth™ could help.